The study of the basic mechanisms of vestibular function.
Team leader
Research topics
Research Topic :
Peripheral vestibulopathies are characterized by unpredictable episodes of vertigo accompanied by postural imbalance and loss of gaze fixation during movement. They are often accompanied by dizziness and nausea. These pathologies can be very disabling, especially when they are recurrent, and can lead to psychological and social isolation. In Europe and the USA, vestibular disorders of peripheral origin represent 3% of consultations of people over 50 and 1% of hospital emergencies. Due to their high prevalence, vestibular disorders constitute a significant burden on our healthcare system. Therapeutic solutions to these pathologies lack specificity and efficacy. This is due both to the lack of knowledge of the pathophysiological mechanisms underlying various vestibular disorders, and to the absence of biomarkers allowing to discriminate between the different types of vestibular damage and to correctly orient therapeutic approaches. Our multidisciplinary research team made up of researchers, lecturer-researchers, engineers and clinicians constitutes a unique environment for studying the basic mechanisms of vestibular dysfunctions, and cognate reactive mechanisms, thus responding to a strong medical need in the field of Neuro-otology, in particular by an approach from bench to bed side.
Scientific guidelines & Strategy
Thanks to its unique position at the interface between fundamental and clinical research, and industrial transfer, the team ambitions to:
• Set up a technological platform, unique in Europe, combining studies on animal models and in dizzy patients
• Decipher the neurobiological mechanisms underlying vestibular disorders and functional restoration
• Develop targeted and more effective therapeutic tools against vestibular disorders
• Promote quality research leading to high-level scientific production
• Become a key player in clinical transfer in order to have a greater impact on the management of balance disorders in patients
• Promote education on balance function and vestibular disorders
• Promote the visibility of research in Neuro-Otology and stimulate the attractiveness of this scientific field in order to encourage the recruitment of the best researchers and students and to obtain academic and private funding
Team Work & Collaborative Environnement
Our team works in a collaborative experimental environment, combining in vitro and in vivo approaches including original study models and methods and technologies available on the site of the Fédération 3C, combined with clinical approaches at the Hôpital de la Conception (Assistance Publique des Hôpitaux de Marseille ; team specialized in medical otology and surgery).
The team's work strategy is based on several axes. On the one hand, the development and functional exploration of animal models of vestibular damage. The search for the pathogenic and neurophysiological supports of the symptoms observed extends down to the cellular and molecular levels, through in vitro approaches on brain slices, explants of vestibular endorgans, and isolated vestibular hair cells and primary neurons. The team benefits from a strong interaction between scientists and clinicians at different levels of the work chain, from the experimental design phase, to potential clinical and industrial transfer. Collaboration with clinicians from the ENT department of the Hôpital de la Conception offers the opportunity, in accordance with good clinical research practices, to verify the validity of new diagnostic tests, and novel therapeutic action of candidate antivertigo drugs.
Main Research Topics
Over the 2018-2023 period, the team is focusing its research activity on several themes:
- the study of the mechanisms of generation and transmission of vestibular sensory information, and the consequences of their deregulation in the generation of vestibular disorders
- the study of the reactive mechanisms evoked following unilateral vestibulopathy and their involvement in subsequent functional restoration
- optimization of investigation methods to better diagnose vertigo syndromes
- multisensory integration and motion sickness
- promotion of research, innovation and transfer
Our team is funder and member of the GDR Vertige (http://gdrvertige.com)
 I-Generation, Encoding and Transmission of Vestibular Sensory Information: Functions & Dysfunctions
I-Generation, Encoding and Transmission of Vestibular Sensory Information: Functions & Dysfunctions
Endolymphatic Homeostasis: control, dysregulation and vestibular disorders
Endolymph occupies a very special place in vestibular physiology and pathophysiology. Unparalleled in the rest of the body, this liquid, whose constitution is highly regulated, behaves as the electrochemical motor of the mechano-electrical transduction process. Many questions remain about the molecular effectors that control ionic endolymph homeostasis and the consequences of their deregulation in both the production of endolymph and generation of vestibular disorders (Maltret et al., 2014). We are currently addressing these questions using an original organotypic culture model of rodent utricle previously developed by our group (Bartolami et al., 2011).
Study of endolymph using the utricular cyst model. The utricular cyst is a unique experimental model developed by our research group. It allows to study the mechanisms of endolymph homeostasis and hydrostatic pressure control. Adapted from Bartolami et al. J Neurosci (2011).
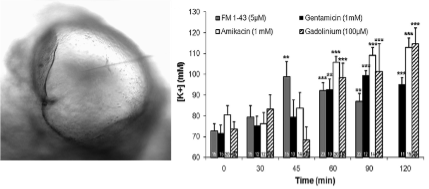
Bartolami S, Gaboyard S, Quentin J, Travo C, Cavalier M, Barhanin J, Chabbert C. Critical roles of transitional cells and Na/K-ATPase in the formation of vestibular endolymph. Journal of Neuroscience (2011) Nov 16; 31 (46): 16541-9.
Maltret A, Wiener-Vacher S, Denis C, Extramiana F, Morisseau-Durand MP, Fressart V, Bonnet D, Chabbert C. Type 2 Short QT syndrome and vestibular dysfunction: mirror of Jervell and Lange-Nielsen syndrome? International Journal of cardiology (2014) 171(2): 291-3.
Chabbert C. Pathophysiological mechanisms at the sources of the endolymphatic hydrops, and possible consequences. J Vestib Res. 2021 Feb 11. doi: 10.3233/VES-200792. PMID: 33579885
Vestibular Neurotransmission: Mechanisms, dysfunctions & vestibular disorders
Over the past two decades, our team has developed several models and methods to study the mechanisms of neurotransmission at vestibular primary synapses. Particular attention was paid to studying the function of the vestibular calyx synapse, a unique synapse found exclusively in the inner ear of higher vertebrates. We were pioneers in demonstrating the functional involvement of glutamate receptors in the calyx neurotransmission (Bonsacquet et al., 2006), and were also the first to report the original mode of glutamate clearance in the calyx synapse (Dalet et al., 2012). Over the period 2018-2023, we intend to continue our investigations in order to identify the molecular effectors (in particular the different types of glutamate receptors) involved in the neurotransmission of vestibular synapses in mammals, as well as their redistribution during selective synaptic damage.
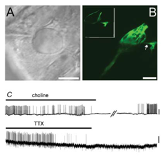
Characterization of neurotransmission at calyx synapses. A. Micrograph illustrating the approach of the patch-clamp pipette through the surface of the posterior ampulla to directly access the calyx terminal for patch-clamp recording. B. Bi-photon imaging of a calyx terminal loaded with Lucifer Yellow while recording. C. Blocking effect of the indicated compounds on the resting electrical activity recorded at a calyx terminal (taken from Bonsacquet et al., 2006)
Dalet A, Bonsacquet J, Gaboyard-Niay S, Calin-Jageman I, Chidavaenzi RL, Desmadryl G, Goldberg JM, Lysakowski A, Chabbert C. Glutamate transporters EAAT4 and EAAT5 are expressed in vestibular hair cells and calyx endings. PLosOne (2012) 7(9):e46261.
Bonsacquet J, Brugeaud A, Compan V, Desmadryl G, Chabbert C. AMPA type glutamate receptor mediates neurotransmission at turtle vestibular calyx synapse. The Journal of Physiology London (2006) 576:63-71.
Tighilet B, Bordiga P, Cassel R, Chabbert C. Peripheral vestibular plasticity and compensation: Evidence and Questions. Journal of Neurology 2019 May 27. doi: 10.1007/s00415-019-09388-9.
II- Adaptive mechanisms involved in functional restoration
Peripheral mechanisms of spontaneous synaptic repair
It is now believed that acute cochlear or vestibular deafferentations could support a majority of auditory and vestibular syndromes such as noise-induced hearing loss and sudden deafness, or labyrinthitis, vestibular neuritis and Ménière's disease. There is currently no targeted pharmacological therapy to effectively repair primary inner ear synapses under pathological conditions. However, an endogenous synaptic self-repair process can take place in the ear of mammals (Brugeaud et al., 2007). This process allows, under certain conditions, to restore hearing and balance. Our project is to explore this original repair processes, to decipher its different phases and to identify the cellular pathways and effectors involved. This is currently what we are working at, through combining histological analyzes of inner ear synapses, molecular studies of gene expression modulation in vestibular sensory epithelia and vestibular primary neurons, following targeted vestibular insults (Cassel et al., 2019).
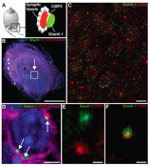
Robust post-lesional plasticity at mammal primary vestibular synapses. In order to mimic the deafferentations of vestibular sensors that occur during peripheral vestibulopathies, we performed excitotoxic-type inner ear deafferentations in rodents by transtympanic administration of glutamate receptor agonists. Analysis of distance between pre- and postsynaptic proteins using by immunocytochemistry has reveal a very high propensity of the primary vestibular synapses to spontaneously reform when hair cells are preserved. This property could be one of the elements that contribute to the spontaneous restoration of vestibular function following peripheral vestibulopathy as observed in humans.
Taken from Cassel et al. 2019 Disease Models & Mechnisms.
Brugeaud A, Travo C, Dememes D, Lenoir M, Llorens J, Puel JL, Chabbert C. Control of hair cell excitability by vestibular primary sensory neurons. Journal of Neuroscience (2007) 27: 3503-3511.
Gaboyard-Niay S, Travo C, Saleur A, Broussy A, Brugeaud A, Chabbert C. Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammal vestibule: an animal model of vertigo symptoms? Disease Models and Mechanisms (2016) 9: 1181-1192.
Cassel R, Bordiga P, Carcaud J, Simon F, Beraneck M, Le Gall A, Benoit A, Bouet V, Philoxene B, Besnard S, Watabe I, Pericat D, Hautefort C, Assie A, Tonetto A, Dyhrfjeld-Johnsen J, Llorens J, Tighilet B, Chabbert C. Molecular and functional correlates of vestibular synaptic de-afferentation and repair in a mouse model of acute onset vertigo. Disease Models and Mechanisms (2019) Jul 15;12(7). pii: dmm039115. doi: 10.1242/dmm.039115.
Mechanisms of Central Compensation
- Demonstration of functional neurogenesis in the vestibular nuclei of adult cats following unilateral vestibular insult.
Following injury of the peripheral vestibular sensors, a spontaneous reactive process referred as Central Compensation allows spontaneous restoration of posture and balance in patients (Lacour and Tighilet, 2010). The efficiency of this process is highly variable and its cellular and molecular mechanisms are still poorly documented. Dr Tighilet was the first to show that the disruption of local homeostasis within deafferented vestibular nuclei promoted reactive neurogenesis in adult mammals (Tighilet et al., 2007) and that this phenomenon was essential for the recovery of balance and gate (Dutheil et al., 2009). We have also shown that GABA is a major regulator of vestibular compensation, not only by coordinating cellular events - including various stages of reactive neurogenesis - but also by regulating the rate of recovery of posturo-locomotor functions (Dutheil et al. al., 2013).
We recently demonstrated that BDNF signaling is involved in the repair neurogenesis induced after vestibular injury, and that specific markers of excitability such as KCC2 transporters and GABAA receptors, locally undergo significant fluctuations in their membrane expression. This suggest that GABA may temporarily acquire a depolarizing role in the vestibular nuclei (VN) during the recovery period (Dutheil et al., 2016). This original plasticity mechanism could partly explain how electrophysiological homeostasis between deafferented and intact VN (considered a key parameter of vestibular compensation) takes place. The current stages of our work consist in studying how the interaction between these cellular effectors promotes the reorganization of neuronal pathways and the restoration of activity in both the ipsilateral and contralateral VN. This is currently achieved by combining cellular and pharmacological studies on models of unilateral vestibular deafferentations in rats and mice, with electrophysiological investigations on brainstem slices. We thus hope to obtain a complete picture of the mechanisms involved in central compensation process, with the ambition of developing targeted pharmacological approaches to accelerate the restoration of balance in humans.
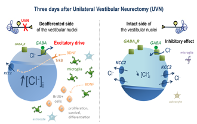
Cascade of events modulating chloride homeostasis in deafferented vestibular nuclei after unilateral vestibular neurectomy. The down regulation in the expression of KCC2 increases in turn cytoplasmic chloride allowing a depolarizing action of GABA. At the same time (3 days after UVN), a peak of BrdU positive cells is observed in the VNs on the injured side, associated with a peak in BDNF expression. This suggests that BDNF, released by neurons and glial cells, could modulate cell proliferation, survival and excitability. Taken from Dutheil et al. J Neuroscience (2016).
Dutheil S, Watabe I, Sadlaoud K, Tonetto A, Tighilet B. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAa receptor in the vestibular nuclei. Journal of Neuroscience (2016) 36(23): 6199-6212.
Tighilet B, Chabbert C. Adult neurogenesis as a toolbox for balance recovery. Progress in Neurobiology (2019) Mar;174:28-35. doi: 10.1016/j.pneurobio.2019.01.001.
- Discovery of a new neurogenic niche in the adult mammals brain
We have just reported for the first time, the presence of neural stem cells (NSC) both in quiescent and proliferating states in the vestibular nuclei of healthy rodents. This suggests the discovery of a new neurogenic niche in the adult mammal brain. In addition, vestibular nerve section (UVN) triggers activation of quiescent NSCs endemic to the deafferented vestibular nuclei. This may provide guiding signals for NSC migration from classical neurogenic niches (subventricular zone and subventricular zone, granular of the dentate gyrus of the hippocampus) to the site of the injury. This original work is now available in a review with a very high impact factor (Rastoldo et al., 2021).
Rastoldo G, El Mahmoudi N, Marouane E, Pericat D, Watabe I, Toneto A, López-Juárez A, Chabbert C, Tighilet B. Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Progress in Neurobiology (2021) Jan;196:101899. doi: 10.1016/j.pneurobio.2020.101899. Epub 2020 Aug 26. PMID: 32858093.
- Role of inflammation in vestibular compensation
Vestibular neuritis, or acute peripheral vestibulopathy, is the second most common case of peripheral vestibular pathologies after BPPV. Although its etiology is still unknown, the hypothesis of inflammation of the vestibular nerve has been proposed for more than 50 years, leading to the prescription of corticosteroids as the gold standard. However, several recent clinical studies question the efficacy of this treatment, and the recent development of transtympanic corticosteroid administrations has not provided the expected benefits. Today many basic questions concerning vestibular neuritis and more generally inflammatory pathologies of the inner ear remain unanswered. One model for studying this pathology is unilateral vestibular neurectomy (UVN) in rodents, which is both a model of post-injury plasticity and a model of central neuroinflammation. An ongoing thesis project in our team aims to elucidate the role of inflammation in this model. This work is carried out in collaboration with Dr Hautefort (APHP), specialist of vestibular neuritis in France. We show in this work that acute treatment with corticosteroids (anti-inflammatory drugs) is deleterious for functional restoration. Our results show a significant decrease in neurogenesis (proliferation, survival and differentiation), in the astrocytic and microglial reaction as well as a significant delay in the restoration of vestibular functions in animals subjected to acute treatment with solumedrol (a drug prescribed in case of vestibular pathologies). An article has just been submitted to the Journal of Neuro inflammation.
Another aspect concerns the role of inflammation in cognition. The objective is to find out whether the pharmacological modulation of inflammation has an impact on cognitive (memory) but also affective and motivational processes that would in turn affect functional restoration in this same rodent model of vestibular pathology. This aspect is original and innovative because it has never been demonstrated. We are collaborating for this work with Pr Sargolini, specialist in spatial cognition.
Nada El Mahmoudi; Guillaume Rastoldo; Emna Marouane; David Péricat; Isabelle Watabe; Alain Tonetto; Charlotte Hautefort; Christian Chabbert; Francesca Sargolini; Brahim Tighilet. Breaking a dogma: acute anti-inflammatory treatment alters both post-lesional functional recovery and endogenous adaptive plasticity mechanisms in a rodent model of acute peripheral vestibulopathy. Journal of Neuroinflammation Submitted. Reference JNEU-D-21-00214.
III. Optimization of diagnostic methods for vestibular syndromes
Treatments of vestibular disorders lack specificity and efficacy (Chabbert, 2013). This considerably slows down the development of targeted therapies adapted to the different types and stages of vestibular disorders. In order to improve the diagnosis of vestibular syndromes, we have initiated a collaborative project involving several members of the GDR Vertige over the period 2018-2023. A first part of the project consists in researching on animal models of vestibular insults new clinical markers of alterations in posture and balance and in developing automated quantification paradigms (Rastoldo et al. 2020; Marouane et al. 2020). A second part of the project, carried out in collaboration with clinicians and vestibular physiotherapists, consists of improving the classification of vertigo in a large population of vertigo patients, with the aim of providing more precise decision-making schemes to rehabilitation practitioners (PhD thesis F Xavier). Thanks to this combined approach, we hope to provide new diagnostic tools for vestibular disorders and thus improve the management of vertigo patients.
Marouane E, Rastoldo G, El Mahmoudi N, Péricat D, Chabbert C, Artzner V, Tighilet B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Frontiers in Neurology. 2020 May 29;11:470. doi: 10.3389/fneur.2020.00470. PMID: 32547480; PMCID: PMC7273747.
Rastoldo G, Marouane E, El Mahmoudi N, Péricat D, Bourdet A, Timon-David E, Dumas O, Chabbert C, Tighilet B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Frontiers in Neurology. 2020 Jun 5;11:505. doi: 10.3389/fneur.2020.00505. Erratum in: Front Neurol. 2020 Oct 26;11:614242. PMID: 32582016; PMCID: PMC7291375.
Xavier F, Chouin E, Montava M, Tighilet B, Lavieille JP, Chabbert C. État des lieux de la rééducation du vertige en France : focus sur la physiothérapie vestibulaire (2020 Report to SFKV; https://www.sfkv.fr ; 2020 Report to SIRV; https://www.vestib.org ).
IV. Multisensory integration and motion sickness
Stéphane Besnard (MD, PhD) is associate professor of Physiology at the Caen University Hospital since 2007. He is associate researcher at UMR 7291 since 2019 and was previously member of INSERM U1075 COMETE for 12 years. He was the head of the medical commission for parabolic flights and in the medical staff, he supervised a research program on motion sickness on Astrolabe spacecraft (IPEV) and on Parabolic flights. His main topics focus on the vestibular system, multisensory integration and motion sickness in patients and in extreme environments, as well as related biotechnological developments. His current research programs focus on the development of a new neurosensory exploration platform in humans applied to vestibular pathology and spatial imbalance / disorientation in healthy subjects exposed to extreme environments (microG, confinement). He works in collaboration with several companies (VIRTUALIS) and is co-founder of two start-ups (VRMaze, Vestia Connect). Since 2010, he has been involved in 10 clinical research programs (PHRC) as co-investigator and 5 as principal investigator. At the start of 2021, he is currently participating in the DeepTime scientific expedition and 4x30 days adaptation.
Besnard S, Tighilet B, Chabbert C, Hitier M, Toulouse J, Legall A, Machado ML, Smith PF. The balance of Sleep: role of the vestibular sensory system. Sleep Medicine Reviews (2018) doi: 10.1016/j.smrv.2018.09.001
5 major publications of the team members
Montardy Q, Wei Mengxia, Tian Yi, Xuemei Liu, Zhou Zheng, Juan Lai, Stephane Besnard, Brahim Tighilet, Chabbert C, Liping Wang. Selective Optogenetic Stimulation of Glutamatergic, but not GABAergic, Vestibular Nuclei Neurons Induces Immediate and Reversible Postural Imbalance in Mice. Progress in Neurobiology (2021 in press).
Rastoldo G, El Mahmoudi N, Marouane E, Pericat D, Watabe I, Toneto A, López-Juárez A, Chabbert C, Tighilet B. Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Progress in Neurobiology 2021 Jan;196:101899. doi: 10.1016/j.pneurobio.2020.101899.
Tighilet B, Chabbert C. Adult neurogenesis as a toolbox for balance recovery: Progress in Neurobiology (2019) Mar;174:28-35. doi: 10.1016/j.pneurobio.2019.01.001.
Besnard S, Tighilet B, Chabbert C, Hitier M, Toulouse J, Legall A, Machado ML, Smith PF. The balance of Sleep: role of the vestibular sensory system. Sleep Medicine Reviews (2018) doi: 10.1016/j.smrv.2018.09.001
Cassel R, Bordiga P, Carcaud J, Simon F, Beraneck M, Le Gall A, Benoit A, Bouet V, Philoxene B, Besnard S, Watabe I, Pericat D, Hautefort C, Assie A, Tonetto A, Dyhrfjeld-Johnsen J, Llorens J, Tighilet B, Chabbert C. Molecular and functional correlates of vestibular synaptic de-afferentation and repair in a mouse model of acute onset vertigo. Disease Models and Mechanisms (2019) Jul 15;12(7). pii: dmm039115. doi: 10.1242/dmm.039115.
Contracts
V. Promotion of Research, Innovation and transfer
In 2016-2018, our team discovered a powerful antivertigo effect of apamin, a specific blocker of small conductance potassium channel activated by calcium (IKCa, SK) in a cat model of unilateral peripheral vestibulopathy (unilateral vestibular neurectomy). Administration of apamin over the first days of vestibular syndrome significantly reduces posturo-locomotor symptoms and nystagmus resulting from the sudden loss of peripheral vestibular sensory inputs. This discovery highlights a pharmacological target which could be exploited as a therapeutic target for the treatment of vertigo attacks linked to peripheral vestibulopathies. In order to protect the pharmacological modulation (inhibition) of SK channels as antivertigo treatment, we filed in March 2018 a patent application (Filing number: PCT / EP2019 / 056342) with the SATT Sud-Est. Following this deposit, a maturation fund was allocated by the SATT Sud-Est to the Physiopathology and Therapy of Vestibular Disorders Team of the UMR7260 CNRS-AMU in order to continue experiments in this field and possibly expand the claims of this patent.
At the end of this maturation phase, the antivertigo effect of apamin initially demonstrated in a cat model (Tighilet et al. 2019) was found in a rodent model of unilateral peripheral vestibulopathy (Tighilet et al., in preparation). We have also demonstrated a very significant antivertigio effect of thyroxine (T4) on a rodent model of unilateral surgical-type vestibular involvement (UVN) (Rastoldo et al., submitted). A patent protecting the use of T4 and its metabolites as an antivertigo drug candidate was filed on June 19, 2019 (EP2020 / 067118). The license of use was acquired by VERTIDIAG, a spin-off of our academic team, in charge of developing these projects (http://vertidiag.com).