Brain, Obesity and diet imbalance
Team leader
Research topics
General theme of the BODI team:
" Bon appétit! We all use this idiom, but do we really know how appetite works? Why can it lead to excessive weight gain? What is the brain contribution to the development of obesity? These are some of the questions our team is trying to answer. We focus on characterizing the neural and glial mechanisms that regulate our eating behavior and that can be altered during pathologies such as obesity, anorexia and diabetes. Our research is carried out in a global context where the obesity “epidemic” is a major public health issue worldwide and has been linked to central leptin resistance induced by excessive consumption of fat.
General physiology and functional exploration approaches, such as feeding behavior, calorimetry, telemetry, forced feeding, stereotaxis and electrophysiology, are tools we use in our explorations as well as different mouse models with diet-induced or genetic energy homeostasis disorders. Cellular and molecular biology techniques complete our scientific analytical set.
Project 1: Astrocytes to fight against obesity?
This project aims to determine the contribution of the glial compartment present in both the hypothalamus and brainstem to food intake and glucose homeostasis regulation. We are developing a multidisciplinary strategy that combines molecular, cellular and physiological approaches in murine models, wild or transgenic. Our goal is to characterize the involvement of glia and gliotransmitters in energy homeostasis and their efficacy towards the treatment of diet-induced obesity. Our project is structured around two main axes:
a- Resistance to leptin as dysfunction of the glio-vascular interface
b- The release of anorexigenic and / or orexigenic substances by astrocytes
Project 2: Nesfatin-1 Neurons: What's the point?
Nesfatin-1, an 82 amino acid peptide derived from the cleavage of NUCB2 protein, exerts an anorectic effect independently of leptin signaling. Although expressed in several peripheral organs, the expression of nesfatin-1 in the brain is limited to a few neuronal groups located in the hypothalamus and the dorsal vagal complex. We want to characterize the contribution, currently poorly understood, of this neuronal population to the regulation of food intake and its disruptions. We have, for example, shown that these neurons are activated in response to an anti-hyperglycemic drug, metformin, and could thus participate in the anorexia induced by this widely used antidiabetic. We have also shown that nesfatinergic neurons are activated in response to various anorectic inflammatory stimuli.
Project 3: Toxins on our plate!
Trichothecenes, toxic metabolites produced by fungi, constitute a global risk for agricultural production and animal and human health. More than 40 countries have introduced regulations or guidelines for levels of contamination for the most common trichothecene deoxynivalenol (DON), on the basis of its deleterious ability to cause stunting. In recent years, considerable research has focused on the mechanisms by which trichothecenes reduce food intake and weight gain. In this context, we have provided significant data on the impact of trichothecenes on the brain, particularly in the hypothalamus and dorsal vagal complex, two structures underlying energy homeostasis. Moreover, our results show that chronic exposure to doses of DON frequently found in the human diet could facilitate obesity by inducing low-threshold inflammation. Low threshold inflammation is considered to promote the progression of various chronic diseases such as diabetes and insulin resistance, atherosclerosis, age-related disorders and neurological afflictions. This hypothesis is currently studied in the team.
Future directions:
In the future, we will expand our research by studying the interactions between nutrition and mental functions. In particular, we will explore the mechanisms involved in learning and memory disorders observed when consuming a fat diet, possibly leading to obesity. We will also analyze the possible causal role of the hippocampus in obesity development and its ability to promote hyperphagia.
 |
 |
 |
|
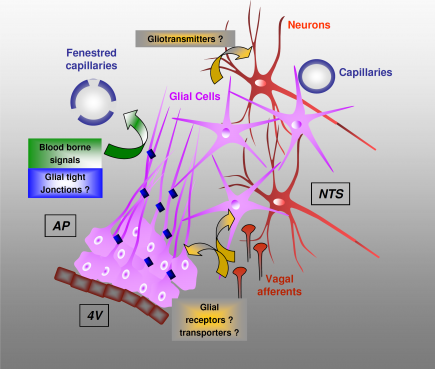
Proposed model of astroglial organization and functioning within |
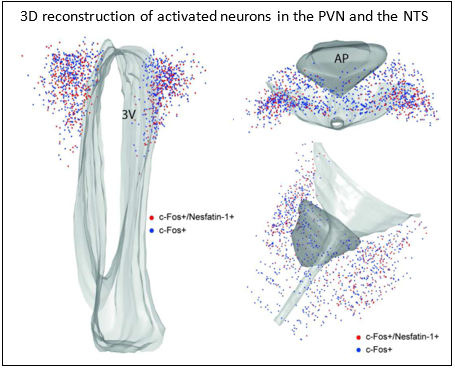
3D reconstructions of c-Fos positive cells in the hypothalamus and |
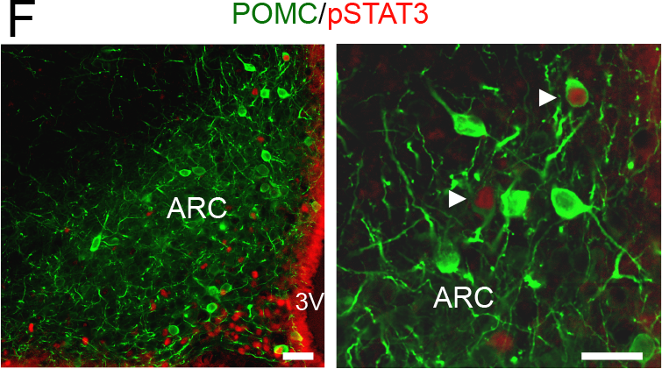
pSTAT3 (red) and POMC (green) double-immunolabeling in the ARC of WT mice treated |